|
The carbonation processes of concrete modelled by the diffusion of
CO2 gas inwards into concrete from the surface under various
conditions are analyzed theoretically based upon unsteady state dynamics,
taking account of the chemical reaction of CO2 with Ca(OH)2
which is assumed to diffuse slowly backwards from the inner layer of
concrete. Analytical solutions were obtained by Laplace transformation
method which involve error functions concerning time and depth from
the surface. For the progress of carbonation (neutralization) of concrete,the
parabolic law concerning time involving constant term 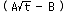 was derived.Making use of this law, the influence of various extrinsic
and intrinsic factors(concentration of CO2 gas, temperature,
water content, and surface mass transfer resistance) on the progress
of carbonation has been evaluated by inserting numerical values into
such material property constants as diffusion constants.Theoretical
results are compared with some field research results. This parabolic
law involving constant term well explains the influence of various factors
on the progress of carbonation.
was derived.Making use of this law, the influence of various extrinsic
and intrinsic factors(concentration of CO2 gas, temperature,
water content, and surface mass transfer resistance) on the progress
of carbonation has been evaluated by inserting numerical values into
such material property constants as diffusion constants.Theoretical
results are compared with some field research results. This parabolic
law involving constant term well explains the influence of various factors
on the progress of carbonation.
Key_Words : Concrete, Carbonation, Neutralization, Prediction, Unsteady State Dynamics, Theoretical Model, Parabolic law
* This paper was revised and reproduced from the manuscript prepared for the Fourth International Conference on Durability of Building Materials and Components, held in Singapore, on November 4-6, 1987.
*1 Head of Inorganic Materials Division of Materials department, Dr. Eng.
|